

ENHERTU was studied in
3 clinical trials of many different
types of metastatic* solid tumors
that had the highest level of HER2 positivity (IHC 3+)
These 3 clinical trials included 192 previously treated adults:
- 111 people with various types of metastatic tumors, including biliary tract, pancreatic, ovarian, cervical, endometrial, bladder, salivary gland, and other cancers
- 17 people with metastatic non-small cell lung cancer (mNSCLC)
- 64 people with metastatic colorectal cancer (mCRC)
These studies only evaluated ENHERTU. There were no comparisons of results to other treatment options. Individual results may vary. ENHERTU may not work for everyone.
ENHERTU was FDA approved for this use based on clinical studies that measured how many patients responded and how long they responded. ENHERTU is still being studied to confirm these results.
*Metastatic is defined as cancer that has spread to other parts of the body.
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.


In the 3 clinical trials, about half of people treated with ENHERTU had their tumors shrink*
*This is called the overall response rate.
In people with various
metastatic tumors,
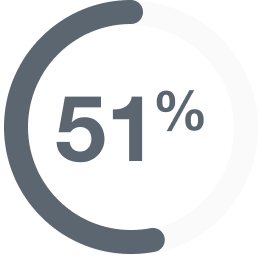
(57 of 111 people)
had their tumors shrink*
- 3% (3 people) had a complete response†
- 49% (54 people) had a partial response‡
- Median duration of response: 19.4 (range: 1.3 to 27.9+) months§
A median is the middle number in a set of numbers.
+ indicates that the response to treatment is ongoing.
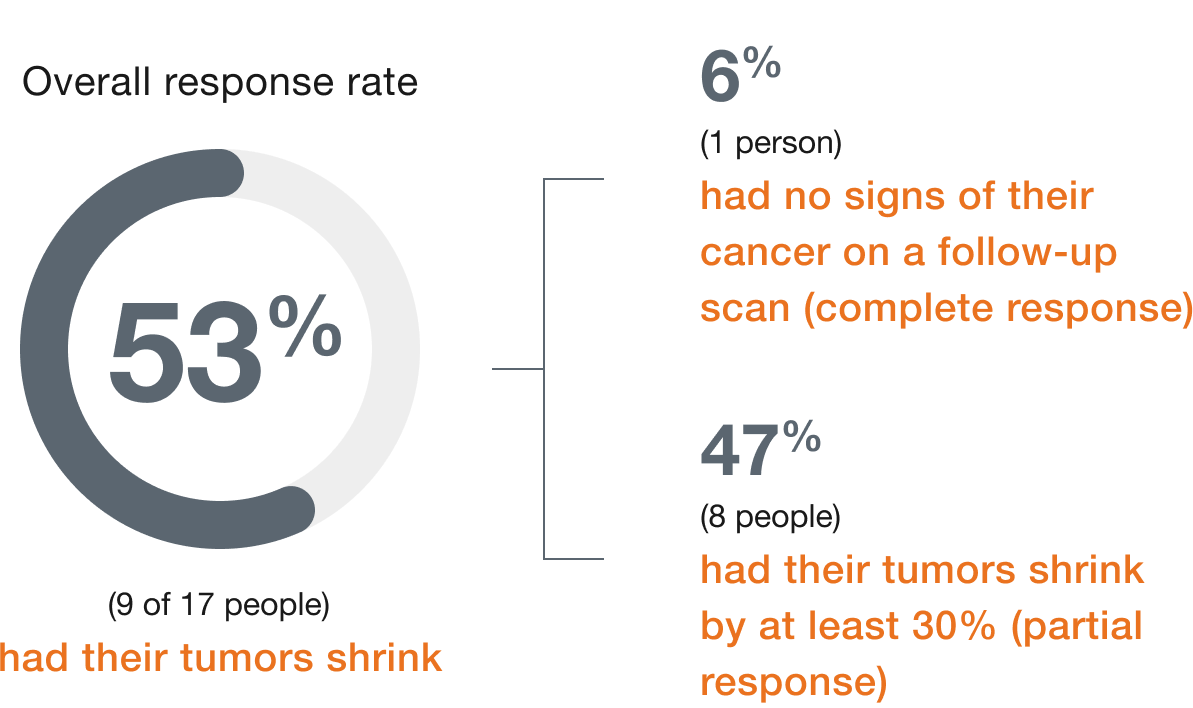
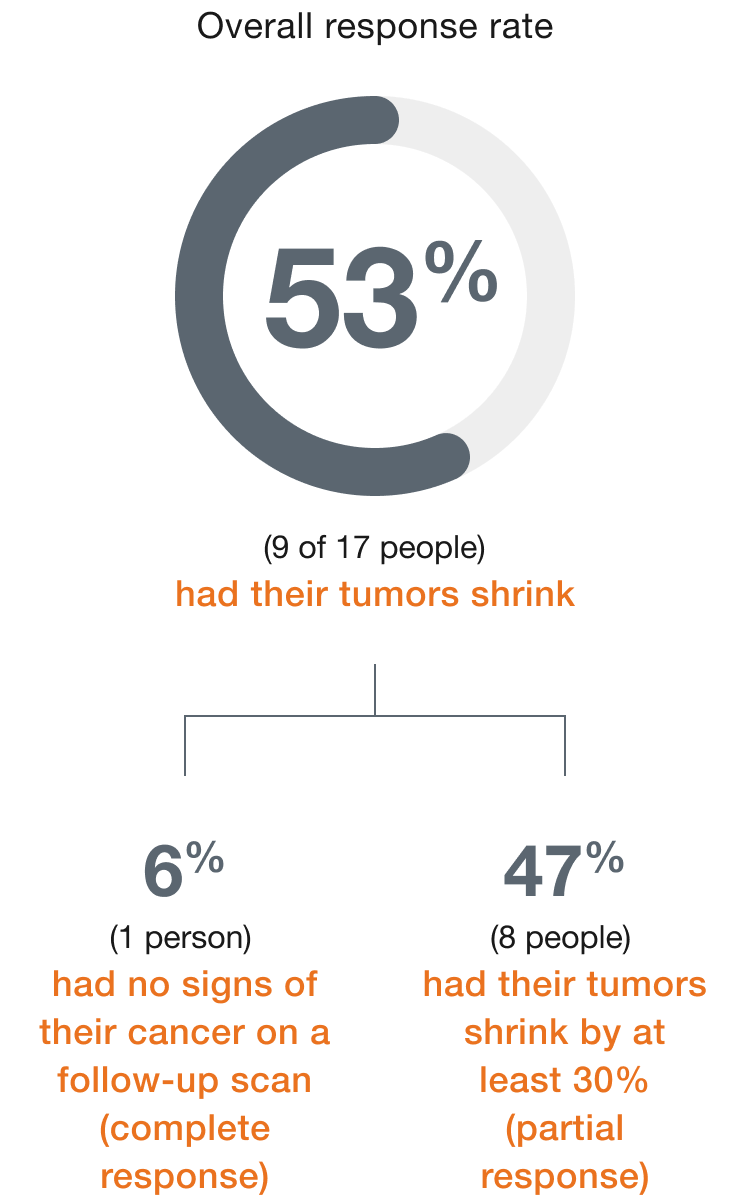
In people with
mNSCLC,
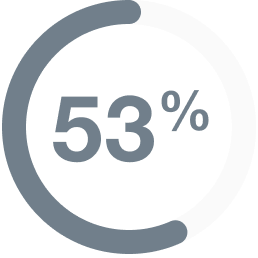
(9 of 17 people)
had their tumors shrink*
- 6% (1 person) had a complete response†
- 47% (8 people) had a partial response‡
- Median duration of response: 6.9 (range: 4.0 to 11.7+) months§
A median is the middle number in a set of numbers.
+ indicates that the response to treatment is ongoing.
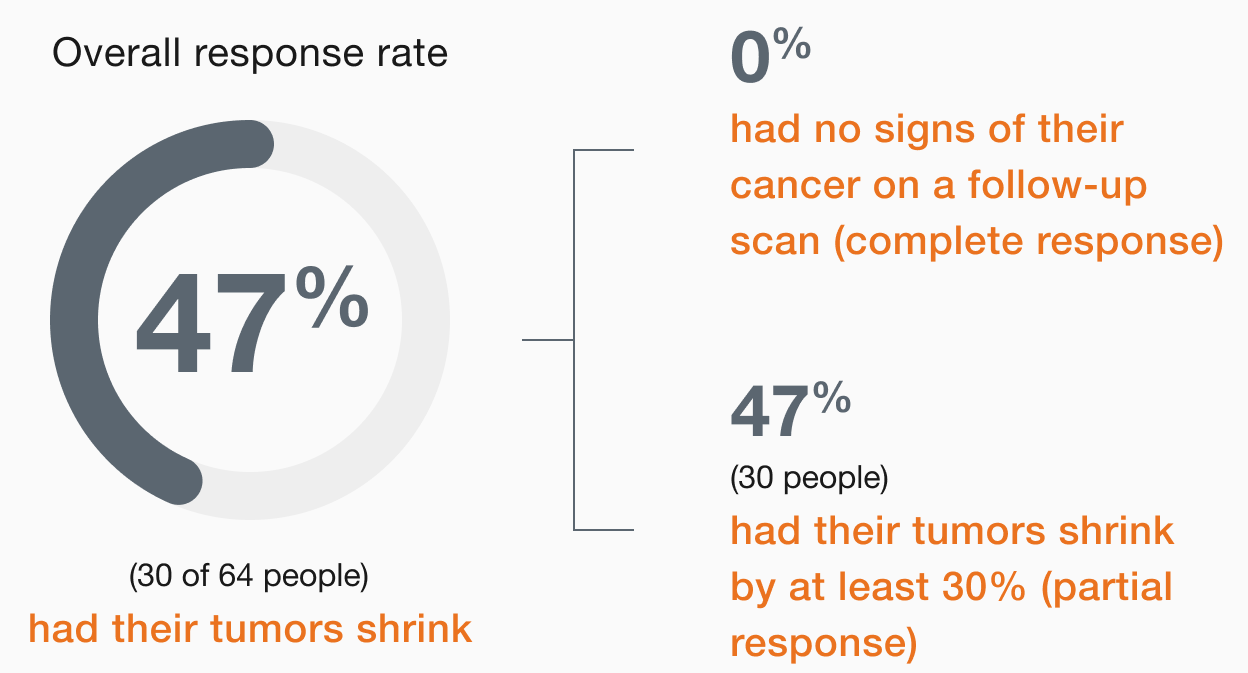
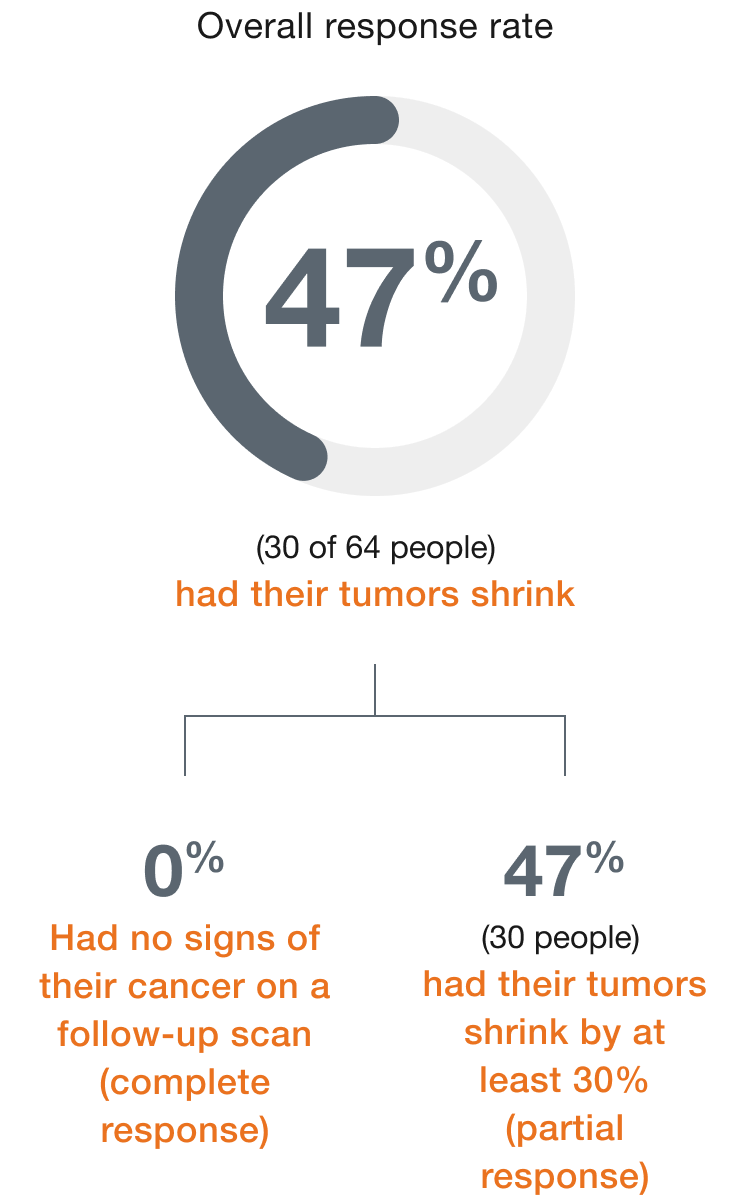
In people
with mCRC,
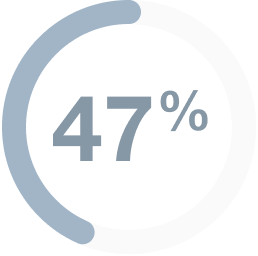
(30 of 64 people)
had their tumors shrink*
- 0 people had a complete response†
- 47% (30 people) had a partial response‡
- Median duration of response: 5.5 (range: 1.3+ to 9.7+) months§
A median is the middle number in a set of numbers.
+ indicates that the response to treatment is ongoing.
†Complete response means there are no signs of cancer on a follow-up scan.
‡Partial response means the tumors shrank by at least 30%.
§Median duration of response is the length of time half the people who responded to ENHERTU continued to respond after the first response was seen.
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; mCRC, metastatic colorectal cancer; mNSCLC, metastatic non-small cell lung cancer.
Results with ENHERTU in people with non-small cell lung cancer
These pages are limited to ENHERTU data in previously treated patients with lung cancer that is HER2 positive (IHC 3+) only; these data do not address all lung cancer clinical trial data for ENHERTU.
In the clinical trial of 17 people with previously treated HER2+ (IHC 3+) mNSCLC, about 50% of people treated with ENHERTU had their tumors shrink
This is called the overall response rate.
50% of people who responded to ENHERTU were responding after 6.9 (range 4.0 to 11.7+) months
This is called median duration of response.*
+ indicates that the response to treatment is ongoing.
*A median is the middle number in a set of numbers. Median duration of response is the length of time half of the people who responded to ENHERTU continued to respond after the first response was seen.
Results with ENHERTU in people with gastrointestinal cancers, including colorectal, biliary tract, and pancreatic cancers
In the clinical trial of 64 people with previously treated HER2+ (IHC 3+) metastatic colorectal cancer (mCRC), nearly 50% of people treated with ENHERTU had their tumors shrink
This is called the overall response rate.
50% of people who responded to ENHERTU were responding after 5.5 (range 1.3+ to 9.7+) months
This is called median duration of response.*
+ indicates that the response to treatment is ongoing.
*A median is the middle number in a set of numbers. Median duration of response is the length of time half the people who responded to ENHERTU continued to respond after the first response was seen.
Outcomes in selected tumor types in the clinical trial of 111 people with various previously treated HER2+ (IHC 3+) metastatic cancers†
Biliary tract (22 people)
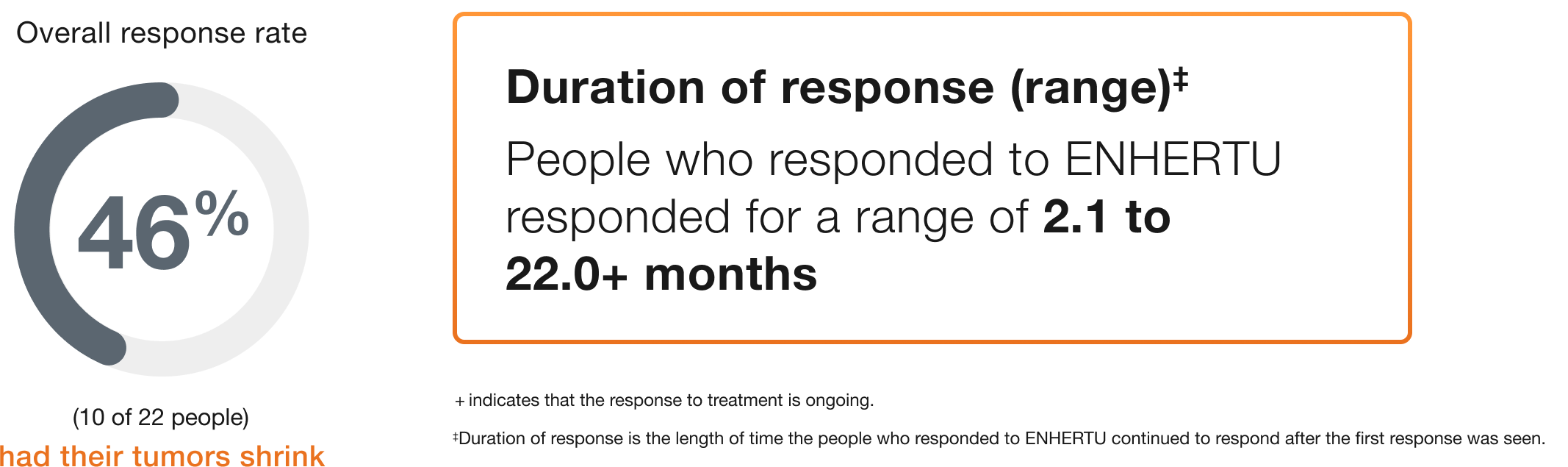
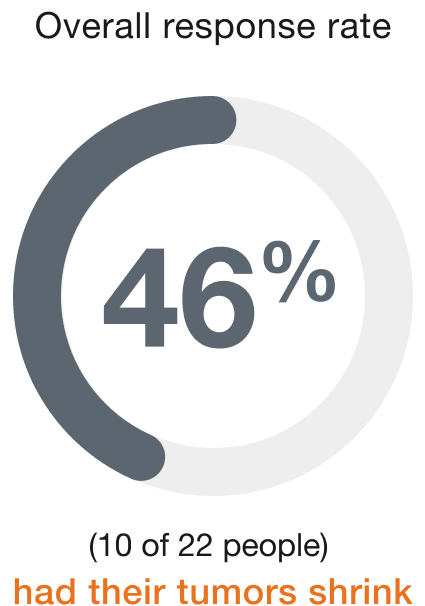
Duration of response (range)‡
People who responded to ENHERTU responded for a range of 2.1 to 22.0+ months
+ indicates that the response to treatment is ongoing.
†Tumor types included biliary tract (22 people), pancreatic (5 people), ovarian (15 people), cervical (10 people), endometrial (16 people), bladder (27 people), and other cancers (16 people).
Pancreatic (5 people)
Overall response rate
0 of 5 people with pancreatic cancer had their tumors shrink
Outcomes in selected tumor types in the clinical trial of 111 people with various previously treated HER2+ (IHC 3+) metastatic cancers*
Results with ENHERTU in people with gynecologic cancers, including ovarian, cervical, and endometrial cancers
Ovarian (15 people)

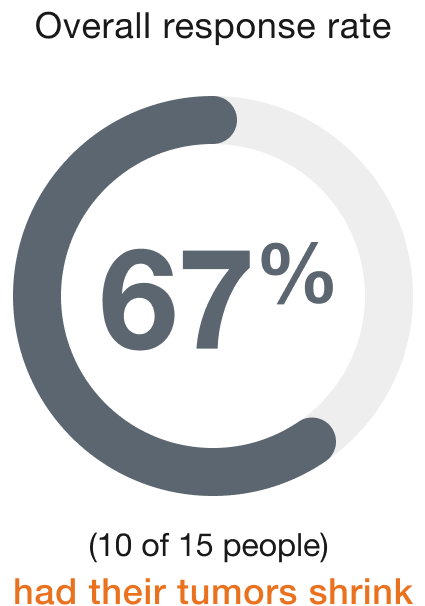
Duration of response (range)†
People who responded to ENHERTU responded for a range of 1.3 to 27.9+ months
+ indicates that the response to treatment is ongoing.
*Tumor types included biliary tract (22 people), pancreatic (5 people), ovarian (15 people), cervical (10 people), endometrial (16 people), bladder (27 people), and other cancers (16 people).
†Duration of response is the length of time the people who responded to ENHERTU continued to respond after the first response was seen.
Cervical (10 people)
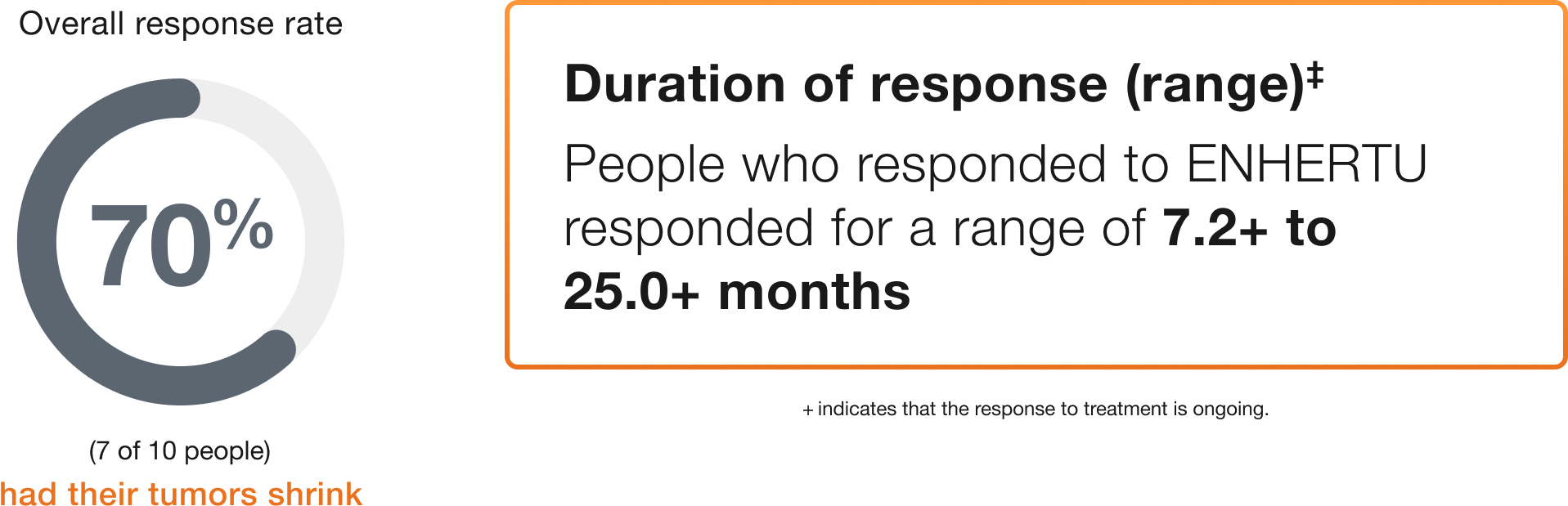
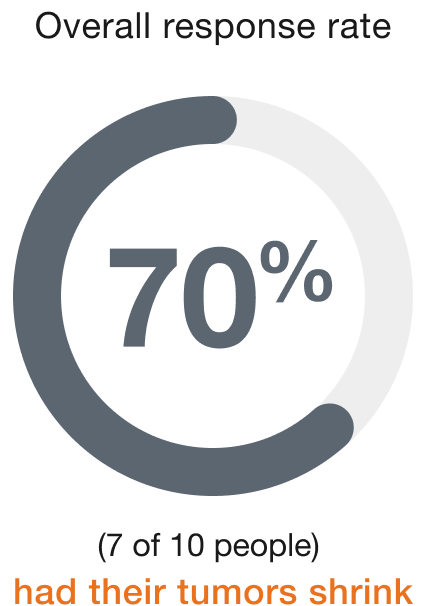
Duration of response (range)‡
People who responded to ENHERTU responded for a range of 7.2+ to 25.0+ months
+ indicates that the response to treatment is ongoing.
‡Duration of response is the length of time the people who responded to ENHERTU continued to respond after the first response was seen.
Endometrial (16 people)
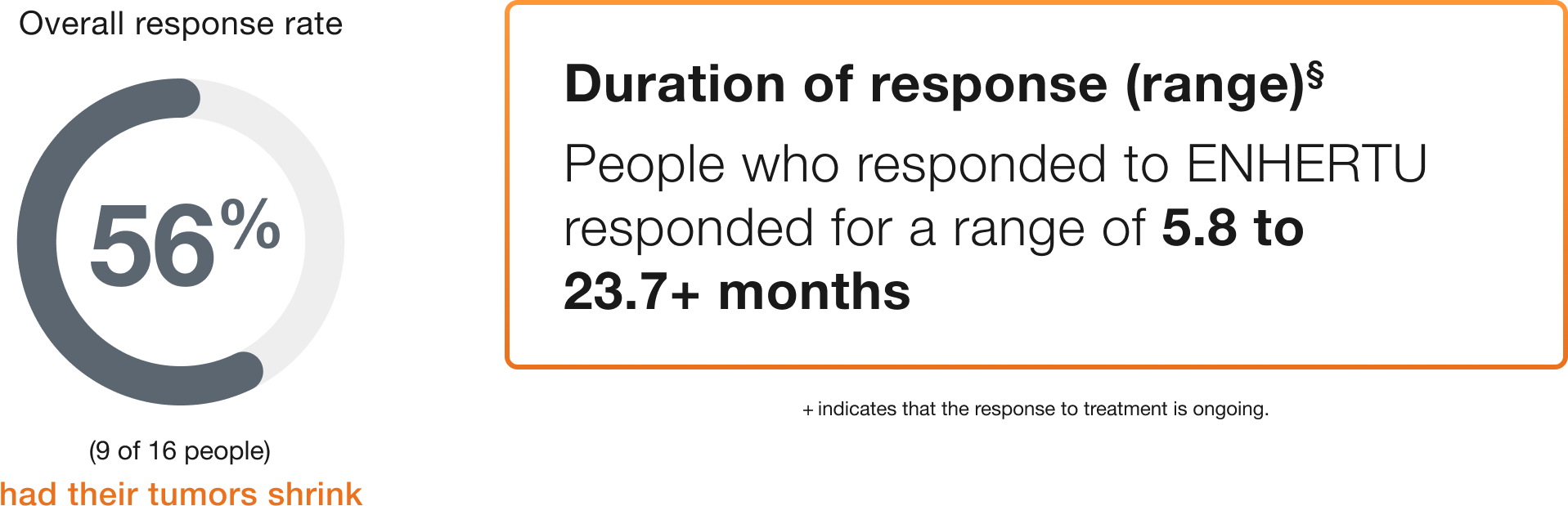
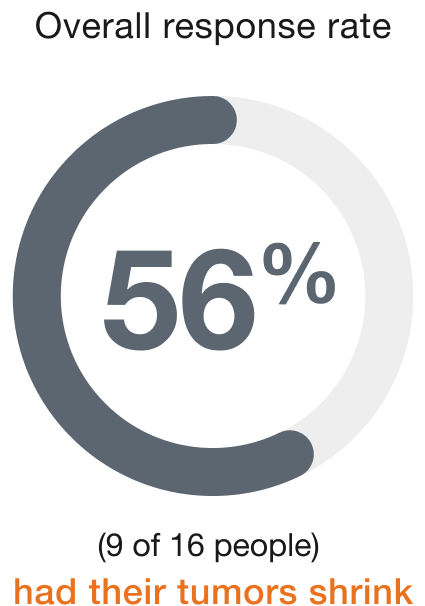
Duration of response (range)§
People who responded to ENHERTU responded for a range of 5.8 to 23.7+ months
+ indicates that the response to treatment is ongoing.
§Duration of response is the length of time the people who responded to ENHERTU continued to respond after the first response was seen.
Outcomes in selected tumor types in the clinical trial of 111 people with various previously treated HER2+ (IHC 3+) metastatic cancers*
Results with ENHERTU in people with bladder cancer
Bladder (27 people)
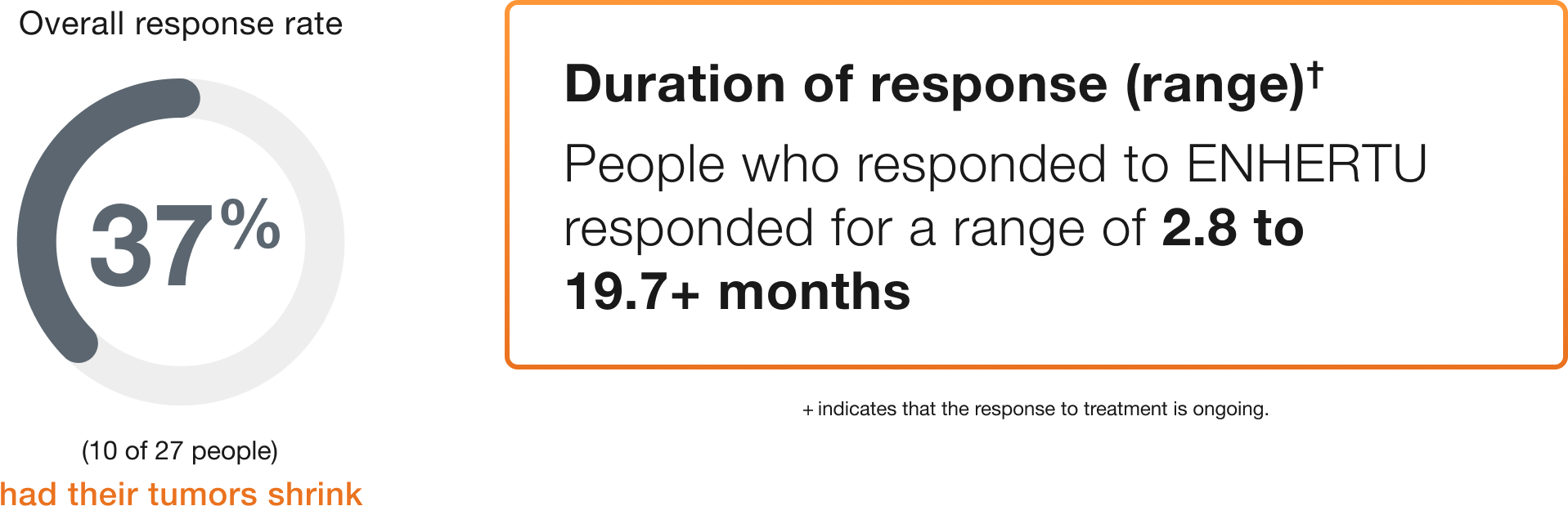
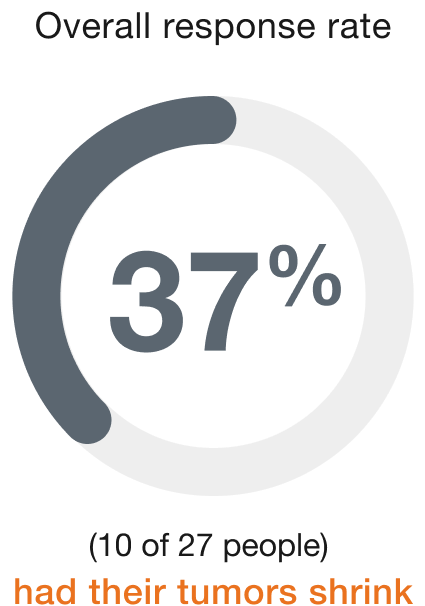
Duration of response (range)†
People who responded to ENHERTU responded for a range of 2.8 to 19.7+ months
+ indicates that the response to treatment is ongoing.
*Tumor types included biliary tract (22 people), pancreatic (5 people), ovarian (15 people), cervical (10 people), endometrial (16 people), bladder (27 people), and other cancers (16 people).
†Duration of response is the length of time the people who responded to ENHERTU continued to respond after the first response was seen.
Outcomes in selected tumor types in the clinical trial of 111 people with various previously treated HER2+ (IHC 3+) metastatic cancers*
Results with ENHERTU in people with salivary gland cancer†
Salivary gland (9 people)

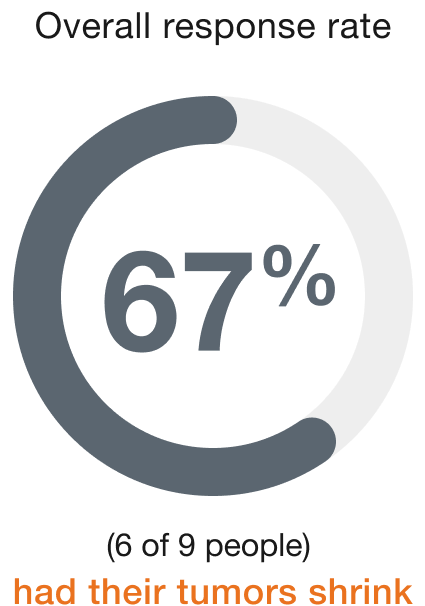
Duration of response (range)‡
People who responded to ENHERTU responded for a range of 5.6 to 20.1 months
*Tumor types included biliary tract (22 people); pancreatic (5 people); ovarian (15 people); cervical (10 people); endometrial (16 people); bladder (27 people), and other cancers (16 people).
†The tumors studied in the group of 16 people with other cancers included salivary gland (9 people); mouth and throat [oropharyngeal] (1 person); vulvar (1 person); skin cancer affecting the sweat glands (1 person); tear gland (1 person); lip/oral (1 person); esophageal (1 person); and esophageal [squamous cell] (1 person).
‡Duration of response is the length of time the people who responded to ENHERTU continued to respond after the first response was seen.
Looking to find resources?
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.

