Two studies evaluated
ENHERTU vs chemotherapy
In adults with HR+, HER2-low or HR+, HER2-ultralow, mBC who received prior hormone treatment in the metastatic setting and have not received chemotherapy for metastatic disease

Up to 90% of people diagnosed with HR+, HER2-negative mBC may have actionable levels of HER2*
*Of 1,856 people with HR+, HER2-negative mBC who were considered to participate in a clinical trial, 1,616 were found to have low levels of HER2.
ENHERTU was compared to chemotherapy in a clinical study of 866 adults who:
- Had low levels of HER2 proteins
- Had received at least one hormone treatment for metastatic disease
Of the 866 adults studied:
436
adults received ENHERTU
430
adults received chemotherapy
713
adults had HR+, HER2-low, mBC
153
adults had HR+, HER2-ultralow, mBC
Of the 713 adults, 359 received ENHERTU and 354 received chemotherapy.
Of the 153 adults, 77 received ENHERTU and 76 received chemotherapy.
Based on the results of the study, ENHERTU is FDA approved for adults with HR+, HER2-low or HR+, HER2-ultralow, mBC who received prior hormone treatment in the metastatic setting.
In adults with either HR+ or HR– HER2-low mBC who received chemotherapy for metastatic disease
ENHERTU was compared to chemotherapy in a clinical study of 557 adults of different ages* and previous treatments who:
- Had low levels of HER2 proteins
- Had unresectable (cannot be removed with surgery) or mBC†
- Had already received chemotherapy for metastatic disease, or had the disease return during or within 6 months of completing adjuvant chemotherapy (after surgery)
Of the 557 adults studied:
373
adults received ENHERTU
184
adults received chemotherapy
494
adults had HR+ mBC
63
adults had HR– mBC
*Patients studied were 28 to 81 years of age.
†Patients with HR+ had received at least one hormonal therapy or were ineligible for hormonal therapy.




In adults with HR+, HER2-low or HR+, HER2-ultralow, mBC who received prior hormone treatment in the metastatic setting
Median progression-free survival
ENHERTU helped people live longer without their cancer growing or spreading compared to chemotherapy*†
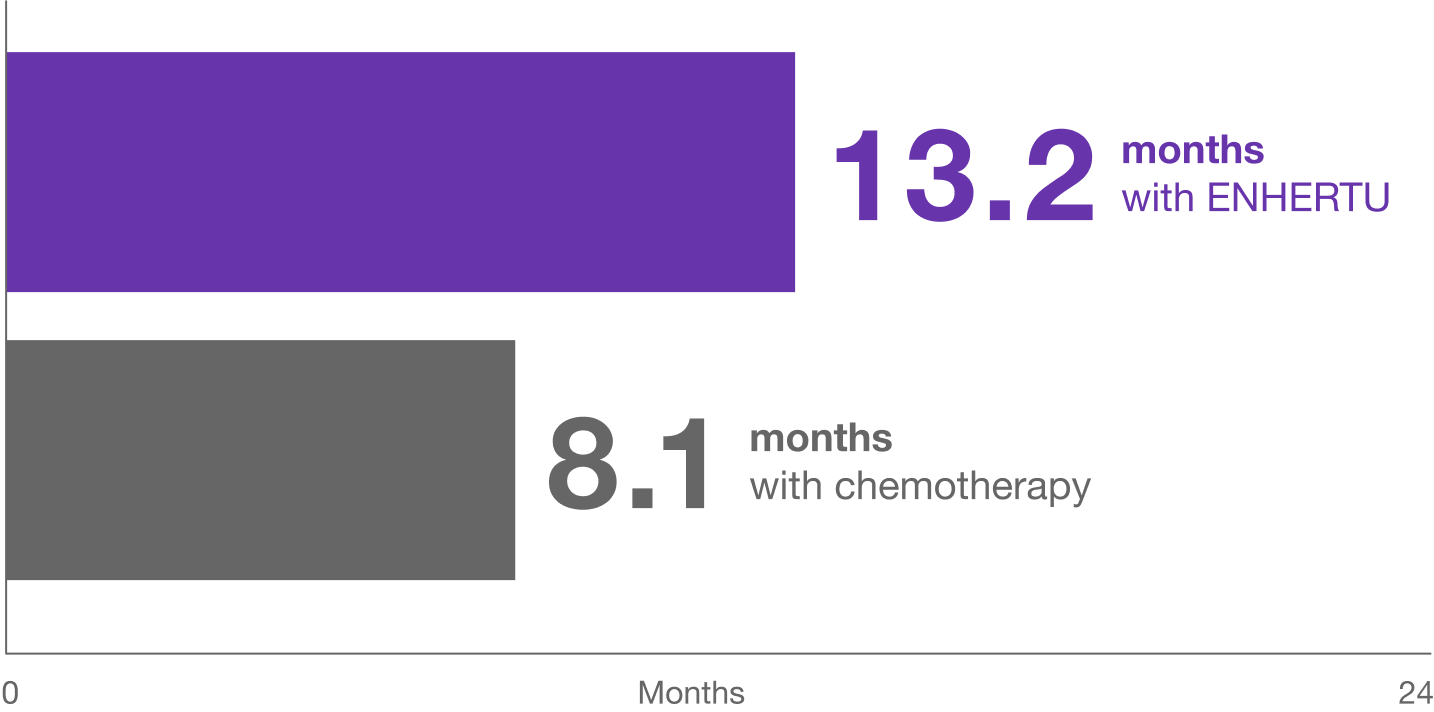

Median progression-free survival is the amount of time that half of the people enrolled in the study were on treatment before their cancer started growing or spreading.
- Primary results for the HER2-low population: 359 people treated with ENHERTU lived a median of 13.2 months without their cancer growing or spreading and 354 people treated with chemotherapy lived a median of 8.1 months
- Exploratory results for the HER2-ultralow population: 77 people treated with ENHERTU lived a median of 15.1 months without their cancer growing or spreading and 76 people treated with chemotherapy lived a median of 8.3 months‡
*Compared to chemotherapy, people who received ENHERTU had 36% lower risk of disease progression or death.
†Patients received physician's choice of chemotherapy. Out of 430 patients, 258 received capecitabine, 103 received nab-paclitaxel, and 69 received paclitaxel.
‡These study results were based on an exploratory analysis, which was not intended to compare the two treatments. The study was also open-label, meaning that both the patients and trial investigators knew which treatment patients received. Therefore, the results could have been influenced by people switching to another treatment, leaving the study, or other factors. This means the results of the exploratory analysis cannot be fully explained and may not be the effect of the treatment. Each person's experience may differ. Speak with your doctor about what you may expect.
Median overall survival
More than half of the people taking ENHERTU were still alive when the latest results were reported
The study is still ongoing. At the time of analysis, more than half of patients taking chemotherapy were also still alive.
Median overall survival is the length of time, from either the date of diagnosis or the start of treatment, that half the people in a group are still alive.
Overall response rate
The percentage of people who had their tumors shrink with ENHERTU and with chemotherapy*†
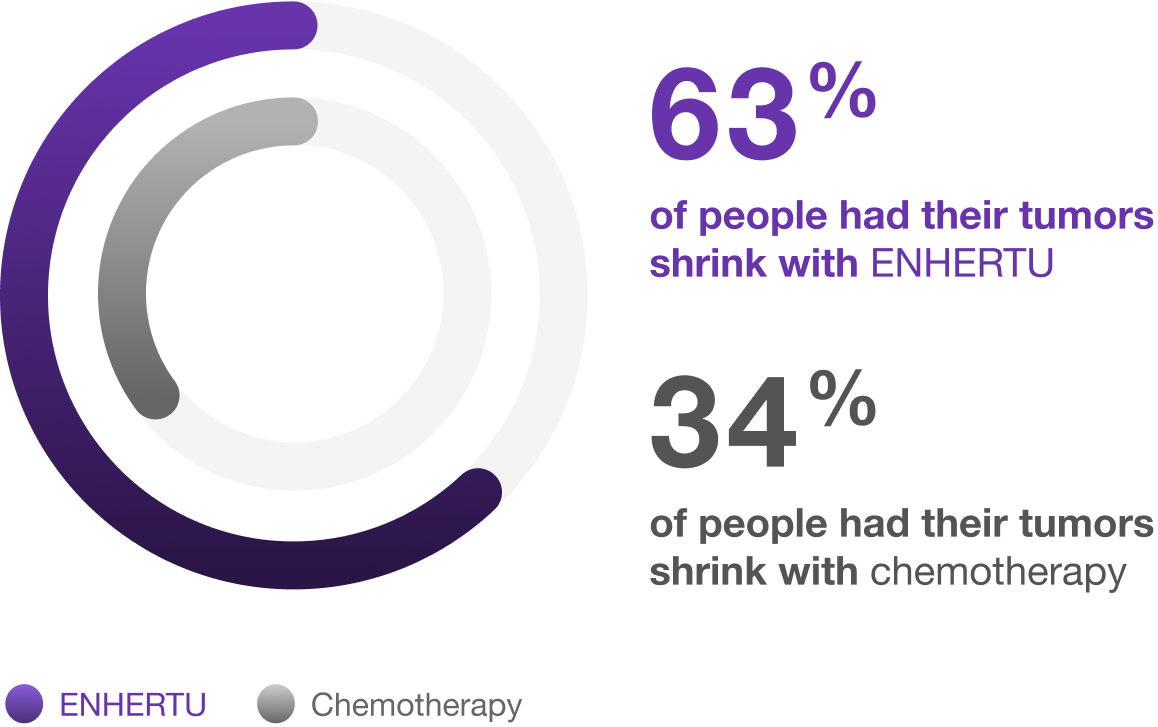
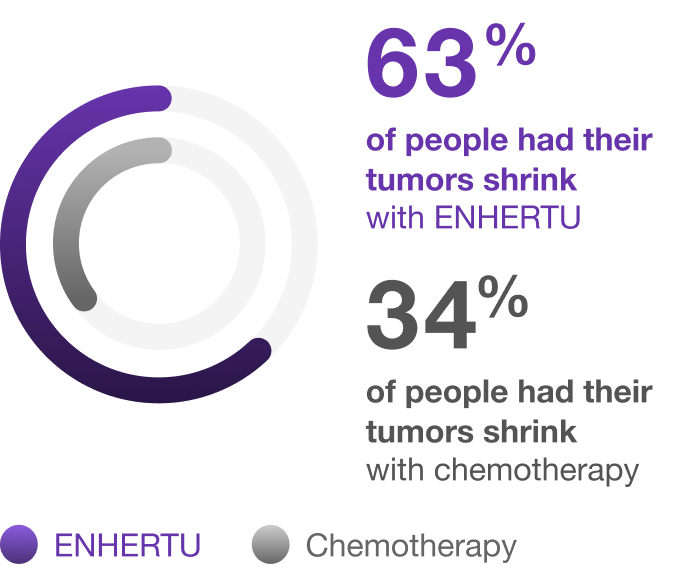
Overall response rate is the proportion of patients who have a partial or complete response to therapy.
- 2.5% (10 of 393) of people treated with ENHERTU and 0% (0 of 389) of people treated with chemotherapy achieved a complete response. A complete response means the tumors could not be seen on imaging tests†
- 60% (236 of 393) of people treated with ENHERTU and 34% (134 of 389) of people treated with chemotherapy achieved a partial response. A partial response means there was at least 30% tumor shrinkage†
- Primary results for the HER2-low population: Based on the people with measurable disease (326 people who received ENHERTU and 324 people who received chemotherapy), 62% (202 of 326) had their tumors shrink with ENHERTU and 35% (114 of 324) with chemotherapy
- Exploratory results for the HER2-ultralow population: Based on the people with measurable disease (67 people who received ENHERTU and 65 people who received chemotherapy), 66% (44 of 67) had their tumors shrink with ENHERTU and 31% (20 of 65) with chemotherapy†
*Based on the people with measurable disease (393 people who received ENHERTU and 389 people who received chemotherapy), 63% (246 of 393) had their tumors shrink with ENHERTU and 34% (134 of 389) with chemotherapy.
†These study results were based on an exploratory analysis, which was not intended to compare the two treatments. The study was also open-label, meaning that both the patients and trial investigators knew which treatment patients received. Therefore, the results could have been influenced by people switching to another treatment, leaving the study, or other factors. This means the results of the exploratory analysis cannot be fully explained and may not be the effect of the treatment. Each person's experience may differ. Speak with your doctor about what you may expect.
Additional results
Disease control rate
With ENHERTU, over 90% of people had their tumors*†:

Shrink
or

Stop growing
Disease control rate is the percentage of people who have achieved complete response, partial response, or stable disease. Stable disease means tumors did not increase in size 20% or more or decrease in size 30% or more.
*92% (362 of 393) of people treated with ENHERTU achieved disease control.
†These study results were based on an exploratory analysis, which was not intended to compare the two treatments. The study was also open-label, meaning that both the patients and trial investigators knew which treatment patients received. Therefore, the results could have been influenced by people switching to another treatment, leaving the study, or other factors. This means the results of the exploratory analysis cannot be fully explained and may not be the effect of the treatment. Each person’s experience may differ. Speak with your doctor about what you may expect.

In adults with either HR+ or HR– HER2-low mBC who received chemotherapy for metastatic disease

Median progression-free survival
ENHERTU nearly doubled the time people lived without their cancer growing or spreading compared to chemotherapy*†
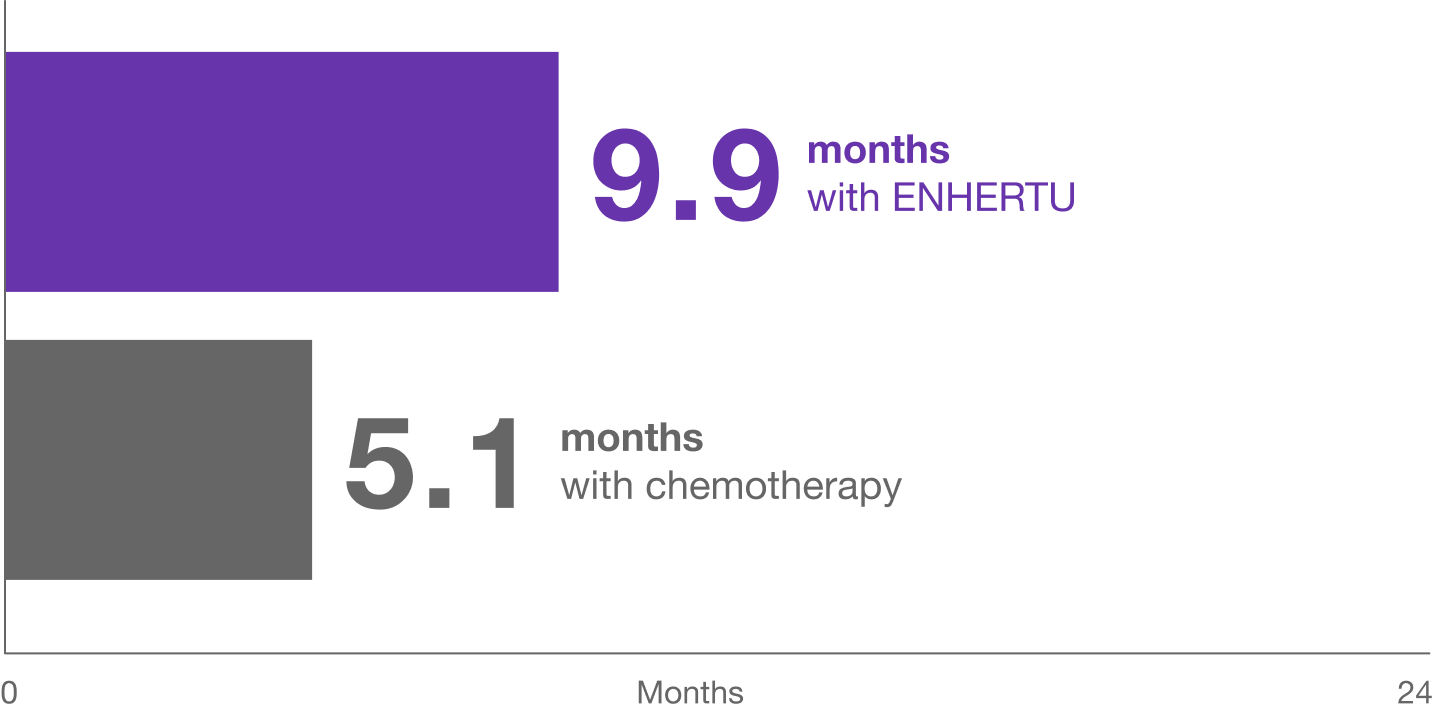

Median progression-free survival is the amount of time that half of the people enrolled in the study were on treatment before their cancer started growing or spreading.
- 331 people who were HR+ and treated with ENHERTU lived a median of 10.1 months without their cancer growing or spreading compared to a median of 5.4 months for 163 people treated with chemotherapy
- The small group of 40 people included in this study who were HR– and treated with ENHERTU lived a median of 8.5 months without their cancer growing or spreading, while the 18 people treated with chemotherapy lived a median of 2.9 months‡
- Before the approval of ENHERTU for people with HER2-low (IHC 1+ or IHC 2+/ISH–) mBC, these people might have been told by their healthcare providers that they had triple-negative breast cancer (ER–, PR–, and HER2-negative)
*35% (130 of 373) of people treated with ENHERTU were alive at the time of data analysis without their cancer progressing, compared to 31% (57 of 184) of people treated with chemotherapy.
†Patients received physician's choice of chemotherapy. Out of 184 patients, 94 received eribulin, 37 received capecitabine, 19 received gemcitabine, 19 received nab-paclitaxel, and 15 received paclitaxel.
‡These study results in HR– patients were based on an exploratory analysis, which was not intended to compare the two treatments. The study was also open-label, meaning that both the patients and trial investigators knew which treatment patients received. Therefore, the results could have been influenced by people switching to another treatment, leaving the study, or other factors. This means the results of the exploratory analysis cannot be fully explained and may not be the effect of the treatment. Each person's experience may differ. Speak with your doctor about what you may expect.
Median overall survival
ENHERTU helped people live longer than chemotherapy*†


Median overall survival is the length of time, from either the date of diagnosis or the start of treatment, that half the people in a group are still alive.
*Patients received physician's choice of chemotherapy. Out of 184 patients, 94 received eribulin, 37 received capecitabine, 19 received gemcitabine, 19 received nab-paclitaxel, and 15 received paclitaxel.
†60% (224 of 373) of people treated with ENHERTU were alive at the time of data analysis, compared to 51% (94 of 184) of people treated with chemotherapy.
Overall response rate
52% of people had their tumors shrink with ENHERTU (16% with chemotherapy)*†‡


Overall response rate is the proportion of patients who have a partial or complete response to therapy.
- 4% of people treated with ENHERTU and 1% of people treated with chemotherapy achieved a complete response†§
- 49% of people treated with ENHERTU and 15% of people treated with chemotherapy achieved a partial response†||
*Patients received physician's choice of chemotherapy. Out of 184 patients, 94 received eribulin, 37 received capecitabine, 19 received gemcitabine, 19 received nab-paclitaxel, and 15 received paclitaxel.
†These study results were based on an exploratory analysis, which was not intended to compare the two treatments. The study was also open-label, meaning that both the patients and trial investigators knew which treatment patients received. Therefore, the results could have been influenced by people switching to another treatment, leaving the study, or other factors. This means the results of the exploratory analysis cannot be fully explained and may not be the effect of the treatment. Each person's experience may differ. Speak with your doctor about what you may expect.
‡52% (195 of 373) of people treated with ENHERTU and 16% (30 of 184) of people treated with chemotherapy had their tumors shrink.
§4% (13 of 373) of people treated with ENHERTU and 1% (2 of 184) of people treated with chemotherapy achieved a complete response.
||49% (183 of 373) of people treated with ENHERTU and 15% (28 of 184) of people treated with chemotherapy achieved a partial response.
Additional results
Disease control rate
Nearly 90% of people treated with ENHERTU had their tumors respond to treatment in at least one of the following ways*†:

Shrink
or

Stop growing
Disease control rate is the percentage of people who have achieved complete response, partial response, or stable disease.
*87% (325 of 373) of people treated with ENHERTU achieved disease control.
†These study results were based on an exploratory analysis, which was not intended to compare the two treatments. The study was also open-label, meaning that both the patients and trial investigators knew which treatment patients received. Therefore, the results could have been influenced by people switching to another treatment, leaving the study, or other factors. This means the results of the exploratory analysis cannot be fully explained and may not be the effect of the treatment. Each person’s experience may differ. Speak with your doctor about what you may expect.
Financial support and downloadable resources
Would you like to receive updates
about ENHERTU?
ER–, estrogen receptor-negative; HER2, human epidermal growth factor receptor 2; HR+, hormone receptor-positive; HR–, hormone receptor-negative; IHC, immunohistochemistry; ISH, in-situ hybridization; PR–, progesterone receptor-negative.


